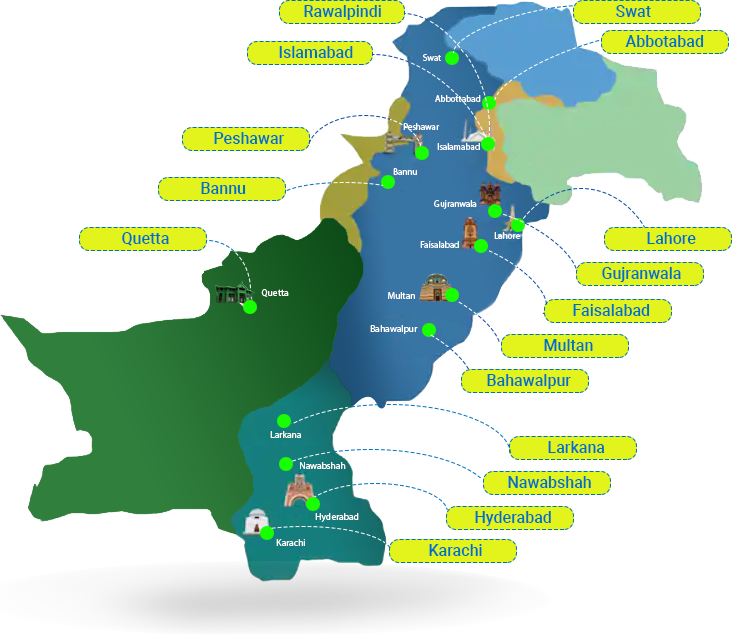
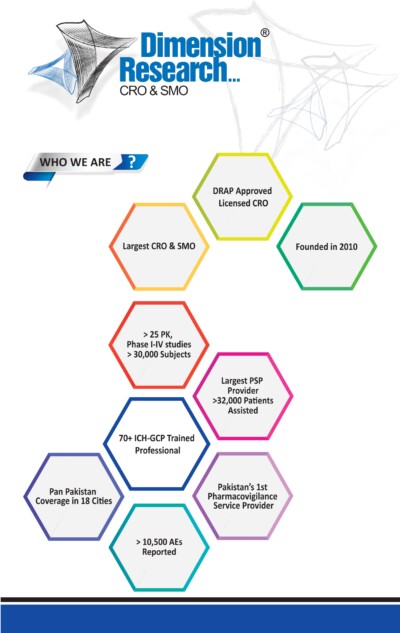
Latest News
Our news center will help you learn more about the latest company activities and media coverage
VISIT OF NUMS FACULTY TO DIMENSION RESEARCH HEAD OFFICE-KARACHI
We are glade to share that National University of Medical Sciences (NUMS) Faculty has visited Dimension Research
World Patient Safety Day Celebration In Dimension Research
We are thrilled to announce that Dimension Research celebrated World Patient Safety Day! Patient safety is a crucial